Nuclear energy is a low-cost and reliable source of energy with a very low carbon footprint, and for these reasons is likely to be a key player in the green energy transition. But, in order to include nuclear energy in our investment plans, we need to ensure the small amount of nuclear fuel waste generated can be stored safely. I am halfway through my PhD project, which is looking at how nuclear waste, stored in geological disposal facilities hundreds of metres below the surface, dissolves when groundwater seeps in through multiple barriers of protection.
Nuclear waste has to be deemed safe for underground disposal for hundreds of thousands, or even millions, of years. Obviously I can’t do experiments over that length of time! But in the lab I try and simulate how waste decays under a range of controlled conditions, asking questions like: What will happen if water breaks through the multiple barriers while the fuel is still very radioactive? What will happen if the water contains different minerals, changing how waste breaks down?
Even though regulators make every effort to ensure this storage is safe over millions of years, scientists have no reference for waste break down over such long periods – so research like mine is critical to understanding how the waste might behave once in the ground.
In this blog post I’ll be talking about the things I love the most about my research: working in the lab and getting to test out new bits of kit. Some of the machines I have used are just astoundingly clever: they can detect tiny details between and inside atoms. They might seem incredibly scientific, but they can paint a picture that is both fascinating and beautiful in its own right.
When I’m in the lab I sometimes feel like I have unlocked a new item on a video game… I can level up my research on a continual quest for more data. The more details I can see, the more clues I can get about the surface and structure of nuclear waste materials, and the better I can understand how they might dissolve once in storage.

I start my experiments by making powder mixtures to replicate common ingredients in nuclear fuel and press it into small pellets about 5 mm across. The next step is a bit more destructive… I heat these pellets at 1600oC (twice as hot as a pizza oven!) so that the powders fuse together and then put them in different solutions to study how they would dissolve in a geological disposal facility for nuclear fuel waste.
It might sound a bit dangerous but don’t worry…for these experiments I use a non-radioactive analogue, cerium dioxide, although once I am better practised, in my PhD, I will get to work on radioactive samples containing uranium and thorium later.
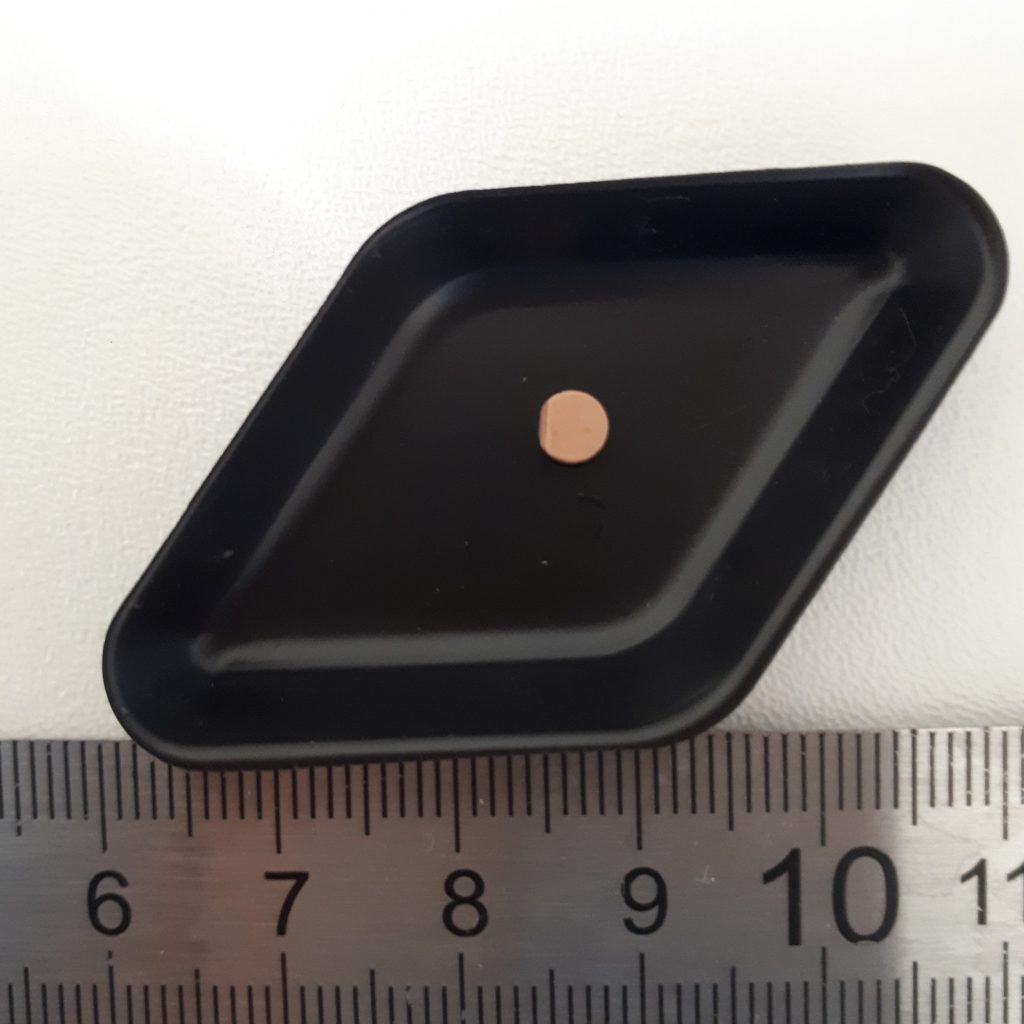
Cerium dioxide pellet 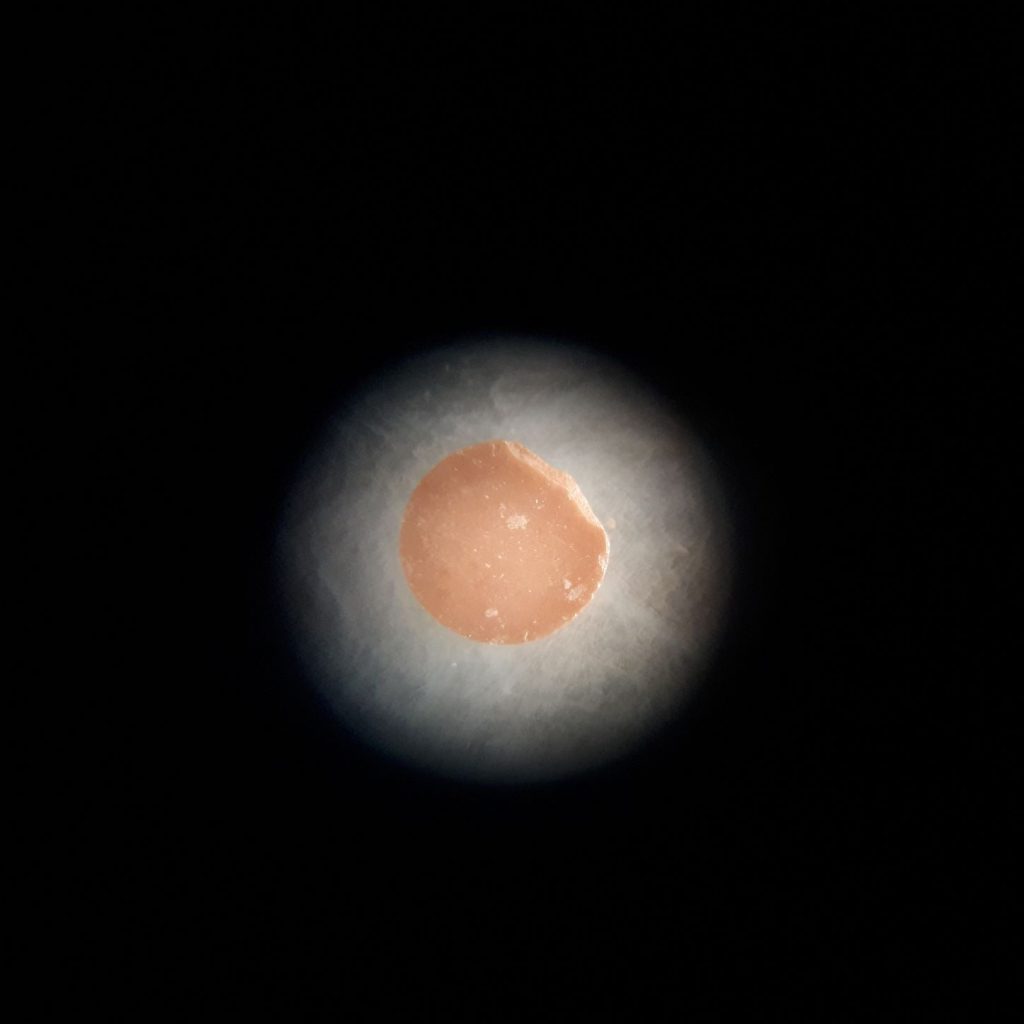
Microscope view of the same pellet 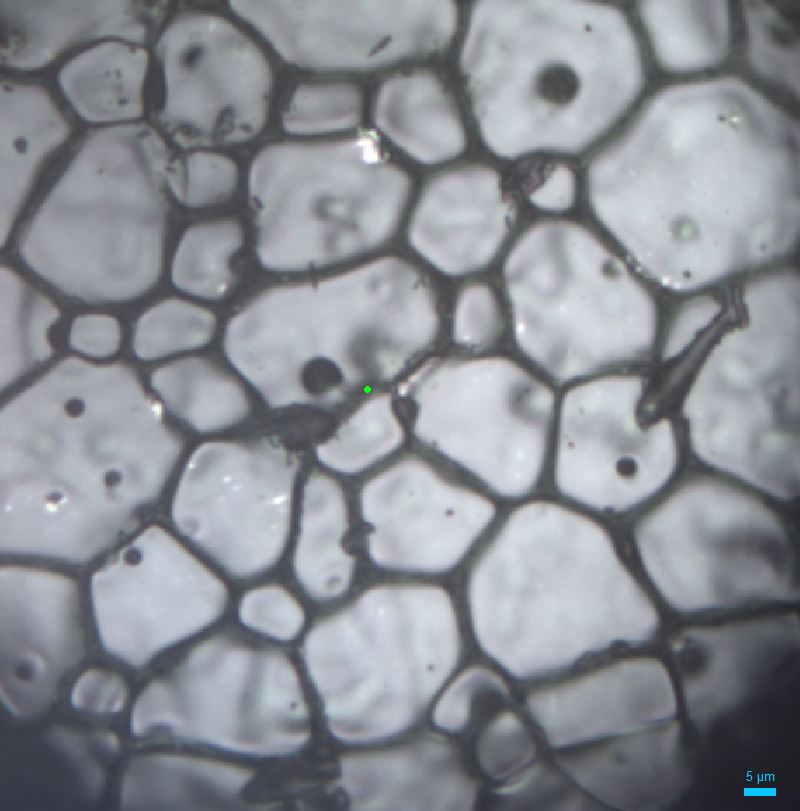
Zooming in at x50 magnification
Once I’ve tried out various leaching experiments on these pellets it’s time to take a look at them under the microscope. With the sample under high power magnification you can see the individual cerium dioxide grains, aligned in patterns and fused together like crazy paving – the average grain size in the images above is about a quarter of the thickness of a strand of human hair!
Standard microscopes work by reflecting visible light of a sample to magnify it, but you can zoom into a surface in even more detail using an electron microscope, which fires a beam of electrons so we can see at nano-dimensions. In the images below you can now see the surface of the cerium dioxide in much more detail, including the small holes and scratches on the surface of the pellet. I must not have cleaned the equipment I use to press the powders very well and accidently scratched them… so now I know to be more careful when I make my uranium thorium dioxide pellets!
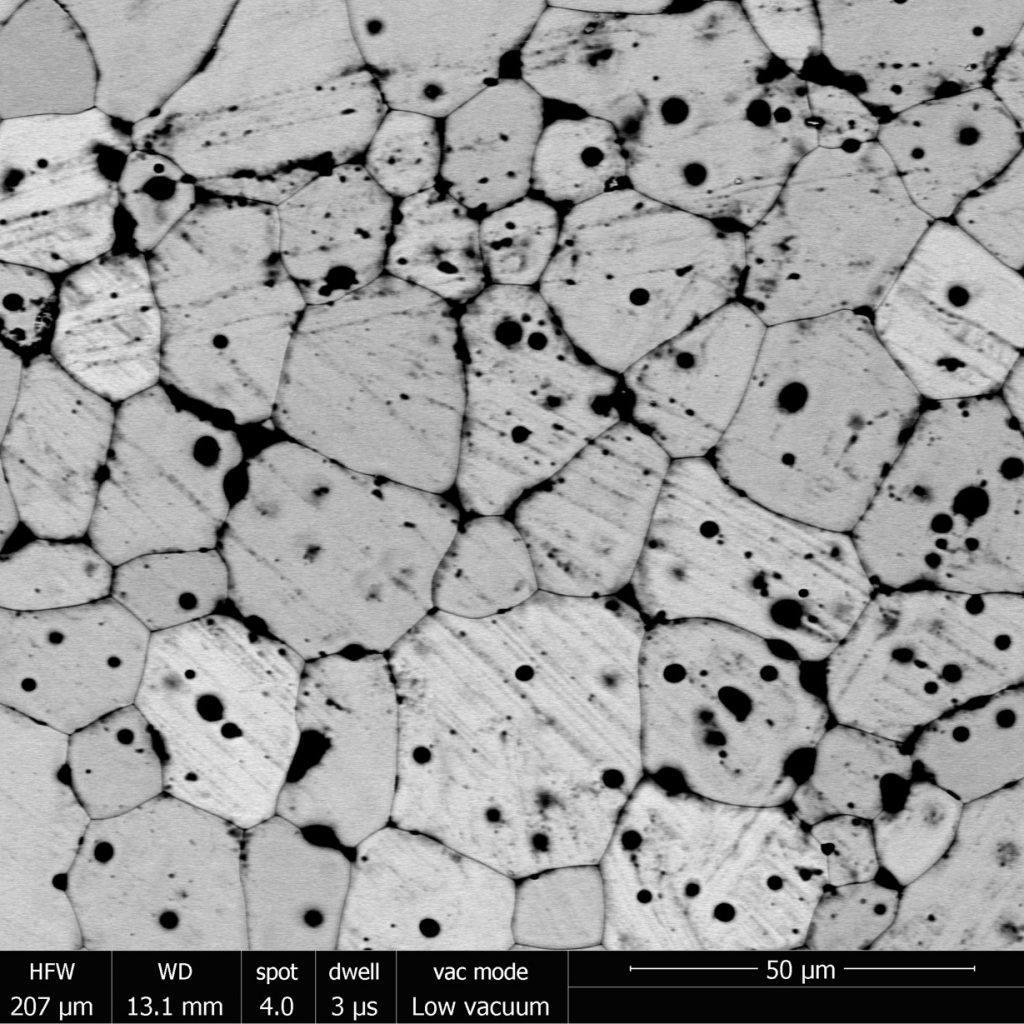
Electron microscope image of pellet’s surface 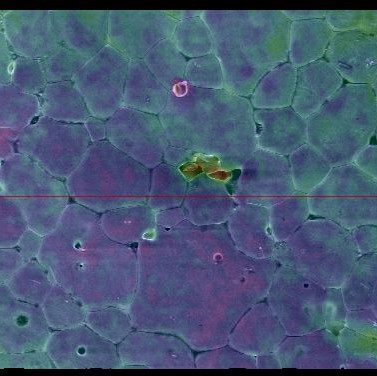
A height map of the sample, produced by tilting it and observing secondary electrons emitted
The electron microscope also allows me to look at the surface using X-rays (as you can see in the first image) to find out how the atoms themselves are arranged and understand strain across the sample. I use this information, together with studying the chemistry of the solutions the samples have been dissolving in, to understand the mechanics of waste breakdown.

One of the most futuristic bits of kit I get to use is Raman spectroscopy, which fires a laser onto a crystal to see right into its structure and map the chemical bonds between the atoms. The results from this have shown me that the cerium in some areas of the samples were bonded to more oxygen. So even though the image above is a good representation of my cerium dioxide pellets more generally, its probable that they contain pockets concentrated in oxygen – meaning they might dissolve slightly differently to real uranium thorium dioxide.
This raises more questions than answers, and might mean I have to expand my search for the right analogue to use in my trials of waste leaching, but that’s what I like about science…I’m on a continual quest to learn more. Maybe one day I will even get the chance to use synchrotron facility…then I could use even brighter light to see these samples even better!
View Emma Perry’s profile and research on our website.


