The Department recently launched its new Part II environmental geochemistry field projects as an alternative to the successful and long-standing mapping projects.
According to Ed Tipper, co-director of undergraduate teaching, “The decision reflects the diverse research areas of our teaching staff, combined with a growing student interest in pressing environmental issues. This year, 13 students enrolled in the new type of project, making it viable to develop a new field trip to train students ready for this environmental pathway.”
The following blog post is written by Tom Marquand, PhD student in the Department and demonstrator on the inaugural environmental geochemistry field trip to Provence, France.
This summer, a pioneering group of incoming Part II students explored mapping through a new geochemical lens. On the new field trip to Provence we showed students vignettes of different geochemistry projects that they could undertake.
Our journey took in the enchanting travertine deposits of the Sainte-Baume National Park found along the River Arc and environmental problems around the brackish lagoon, Étang de Berre.
At our first stop, the students were wowed by the travertine springs emerging from the Mesozoic limestones, where they diligently sampled, probed and titrated the course of two streams.


We measured sharp increases in pH as the surfacing water quickly degassed its supersaturated carbon dioxide content. Corresponding changes in alkalinity and total dissolved solids helped to confirm the presence of authigenic carbonate precipitation and even indicated the presence of hidden springs disguised from view under the stream itself.
We were impressed by the self-motivation of the students, who sampled the length of the River Arc. Working as a team, they prioritised the stops, navigated to them, and worked together to sample and divide up tasks in order to maximise the data they could collect.



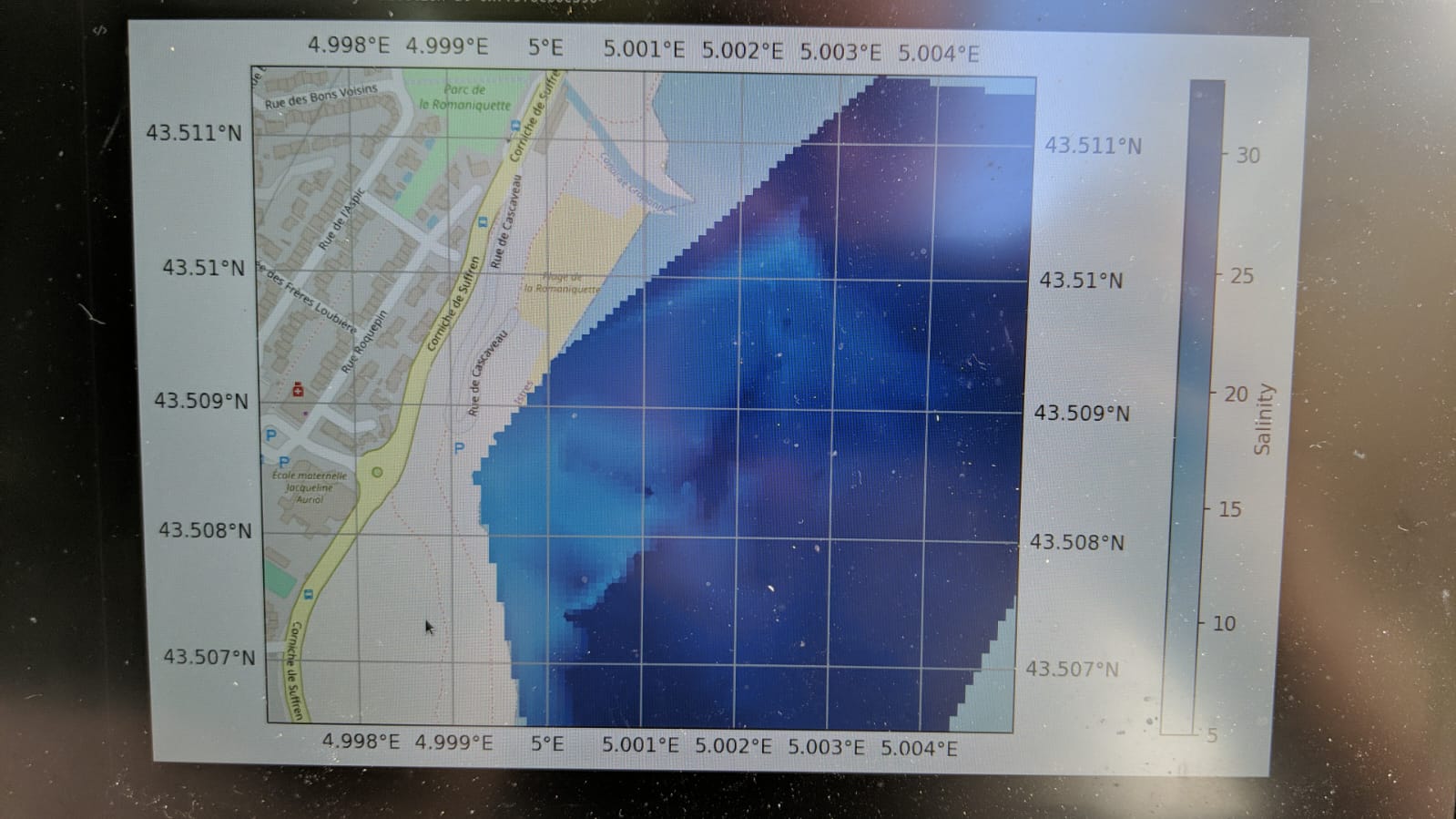
Then, taking kayaks and a motorboat out into the Étang de Berre, the students were able to capture the dispersal of a freshwater pool where agricultural wastewater was pouring out into the lagoon.
The detailed map of the plume facilitated a discussion about the flawed placement of the swimming barrier at the site, which tended to trap the wastewater in the swimming area. At some parts of the plume it was even possible to see a degree of stratification when different depths were sampled at the same location.


Each evening, the group returned to the field lab where they demonstrated their proficiency in a number of spectrophotometric assays. Using the Department’s new portable spectrophotometers and test kits, students measured concentrations of major elements and pollutants.
We were pleased to see generally low pollutant concentrations, though some sites with elevated pollutant levels suggested a significant input of wastewater or agricultural runoff.

All of this was overseen by the brilliant teams of lecturers and demonstrators who I was very lucky to work with: Marie-Laure Bagard, Oscar Branson, Emily Stevenson, and Ed Tipper.
For the amazing success of this new trip, we owe our thanks to the hard work of Ed and Emily who carefully planned and organised the schedule and equipment such that no one would guess it was a brand-new programme.
This trip, and the associated geochemical mapping projects, will provide students with a new perspective and skill set for understanding important chemical processes in the critical zone while still allowing them to develop the planning, interpretation, and analytical skills that the traditional mapping project provides.
We hope this trip attracts more students to our exciting department; after all, the scholarly diversity of Earth Sciences is one of its greatest strengths.



Super initiative and a good field trip report to explain some of the ideas implemented.